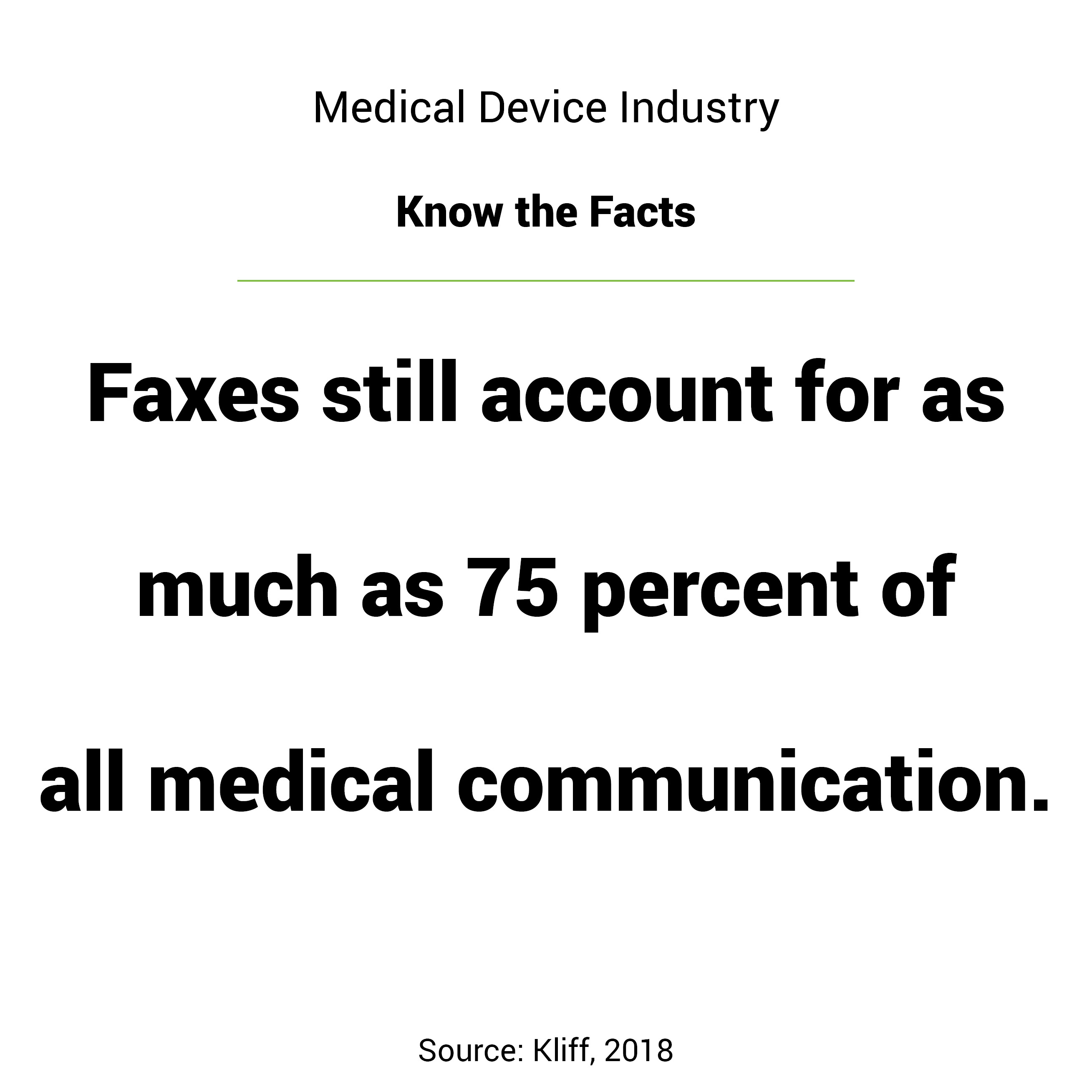
Novasyte is a Sponsor of this year's Field Service Medical, the Medical Device Service Conference, happening in San Diego, CA from Monday, February 25 through Wednesday, February 27. The conference is for MedTech leaders focused in customer success, service and support.
A few of our team members will be at the show, including including Hillary Medina, our Vice President of Field and Recall Solutions, Heidi Porter, our Director of Sales - Northwest, and Stephanie Kaderli, our Director of Sales - Southwest.