When it comes to electronic medical records, several words beginning with ‘F’ may come to mind.
You may be surprised to learn that, according to an article by Sarah Kliff, faxes still account for as much as 75 percent of all medical communication (Kliff, 2018). This is due to several factors. One of the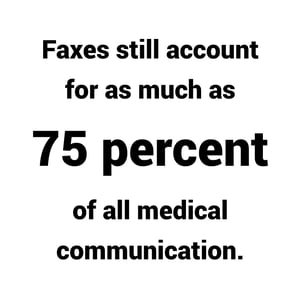 main drivers is the lack of interoperability between competing electronic medical records systems.
main drivers is the lack of interoperability between competing electronic medical records systems.
So the fax machine survives, even though it is a dated technology widely despised by stakeholders throughout the healthcare spectrum.
Advantages to the fax machine include that it doesn’t require a login to access nor does it need months of training to operate. However, while these can be seen as benefits for end users, challenges arise when analyzing the security, traceability and confirmation of faxed documents.
Kliff asserts a major drawback to faxes is they can end up at the wrong location. “One medical worker recalled a fax fiasco from the 1990s when he practically sent medical records to the moon,” (Kliff, 2018).
We have come face-to-face with the fickle fax culture through many medical device recalls over the past 11 years. We found the same challenges arose when notifying consignees and collecting their responses. When managing a recall, it is important a facility responds appropriately, acknowledges they are aware of the action and takes the appropriate steps to remedy the issue. This often means to read the recall notice and complete the enclosed green card.
The green card is used to serve the ensuing purposes:
- Confirm the consignee information (account number, street address, etc.)
- Collect the respondent’s information (who filled it out)
- Collect the product disposition information
- Depending on the action required, a facility may need to ship the product, request a service appointment or simply acknowledge they do not have it anymore.
All of this information is critical when it comes to a manufacturer and a consignee resolving a field action. As it stands today, the process often goes as follows:
Manufacturer issues their field action → Sends out a letter to all impacted consignees with corresponding green cards → Consignees receive the letter, fill out the green card and either fax or mail it back → Manufacturer then takes the faxed responses, transcribes the information and takes action from there.
So, what’s the issue?
In order for those steps to take place, it can take weeks or months to complete via fax and consequently delays the entire recall process.
Here at Novasyte, we still battle with the fax machine every day. So far, we are winning. Our S.M.A.R.T. Response Program addresses the hindrance and data accuracy concerns caused by managing a medical device field corrective action or recall through a fax machine.
Customers and consignees appear to love our approach. Here is some recent feedback:
- “Novasyte accomplished in six weeks what would have taken us six months.” - VP of Sales
- “As I informed the rep I worked with, this is the fastest turn-around I’ve had with a recall since taking this position.” - Senior Director of Recalls
- “This is by far the easiest method of handling recalls. It is fast and convenient.” - Inventory Control Lead
- “I have been doing recalls for the past 16 years and this is the easiest and best process I have worked with to-date. GREAT JOB!” - Assistant Director of Supply Chain Operations
If you’re interested in learning more, we encourage you to download a recently released case study regarding a MedTech recall that affected 1 million units and 3,200 consignees across the U.S.
Three particularly interesting results by utilizing the S.M.A.R.T. technology:
- Time to launch: Reduced from 10 days to 5 days
- Time to achieve 100% due diligence: Reduced from 7 months to 2.5 months
- Time to achieve 80% consignee response: Reduced from 10.5 months to 6 months
If you want to learn more, click on the link below or watch this 60-second video.