Novasyte Health, an IQVIA™ company, is proud to announce that we have been named one of San Diego Business Journal's (SDBJ) 2019 Best Places to Work.
"We are honored to receive this recognition from San Diego Business Journal,” said Tim Gleeson, vice president and general manager at Novasyte Health, an IQVIA™ company. “At Novasyte, we pride ourselves on the caliber of our people, core values, culture, and our ability to provide superior services for our clients.”Novasyte Health Named One of SDBJ’s Best Places to Work in San Diego 2019
Topics: Novasyte News, Novasyte Growth, Med-Tech Industry Update, Novasyte Health, an IQVIA™ company
A Career Where You Travel: The Top 3 Most Visited Cities by Novasyte Health Employees
Do you love to travel? Have you always wanted to visit some of the best cities in America but are too busy with work to plan the trip?
As a leader in the MedTech industry, Novasyte Health employs a network of more than 2,200 healthcare professionals across the U.S. and Canada, and we are continually recruiting for new positions. One of the best perks of being a Novasyte Health employee is the opportunity to travel for assignments.
Topics: Outsourcing Support, Med-Tech Industry Update, Medical Device Industry, Industry Trends
Novasyte Health Awarded One of Eight Global Suppliers of the Year by Becton Dickinson
Novasyte Health is excited to announce that we have been named one of eight Suppliers of the Year by Becton Dickinson (BD) at the annual Supplier Recognition Awards that took place on March 12, 2019. This marks an important milestone in our long-standing partnership with BD.
"We are thrilled to receive the honor from BD and look forward to the efforts we will both make toward our mission of improving the lives of patients," said Novasyte CEO and Chairman, Tim Gleeson.
Topics: Novasyte News, Novasyte Growth, Med-Tech Industry Update
5 Reasons to Join Novasyte’s Clinical Nurse Educator Team
In the first months of the new year, people are often looking for a change. There are three common changes in the minds of many professionals, including lifestyle, working environment and potentially, employment.
If you are a Registered Nurse (RN) interested in opportunities away from traditional bedside care, Novasyte can offer all of those things. Novasyte partners with medical device companies to assist them with a variety of product commercialization services, including product education. Today, we hire hundreds of RNs as Clinical Nurse Educators. They travel the country and teach their fellow nurses how to safely use new, cutting-edge technologies.
Topics: Outsourcing Support, Med-Tech Industry Update, Medical Device Industry, Medical Device Field Action, S.M.A.R.T Programs, Industry Trends, SMART recall program
MedTech Recalls: What Preliminary Questions Should You be Asking?
Field actions happen; even to the most diligent organizations.
Organizations across the medical device manufacturing industry understand this as a sharp reality. Over the last decade, however, product recalls have substantially increased and are reaching record highs. Over 208 million devices were recalled in the beginning of 2018, more than the total number of recalled devices in all of 2017.
Topics: Outsourcing Support, Med-Tech Industry Update, Medical Device Industry, Medical Device Field Action, S.M.A.R.T Programs, Industry Trends, SMART recall program
Novasyte is a Sponsor of Field Service Medical 2019
Novasyte is a Sponsor of this year's Field Service Medical, the Medical Device Service Conference, happening in San Diego, CA from Monday, February 25 through Wednesday, February 27. The conference is for MedTech leaders focused in customer success, service and support.
A few of our team members will be at the show, including including Hillary Medina, our Vice President of Field and Recall Solutions, Heidi Porter, our Director of Sales - Northwest, and Stephanie Kaderli, our Director of Sales - Southwest.
Topics: Novasyte News, Medical Device Recall, Medical Device Recall Help, Med-Tech Industry Update, Medical Device Industry, Medical Device Field Action, S.M.A.R.T Programs, SMART recall program, Field Technical
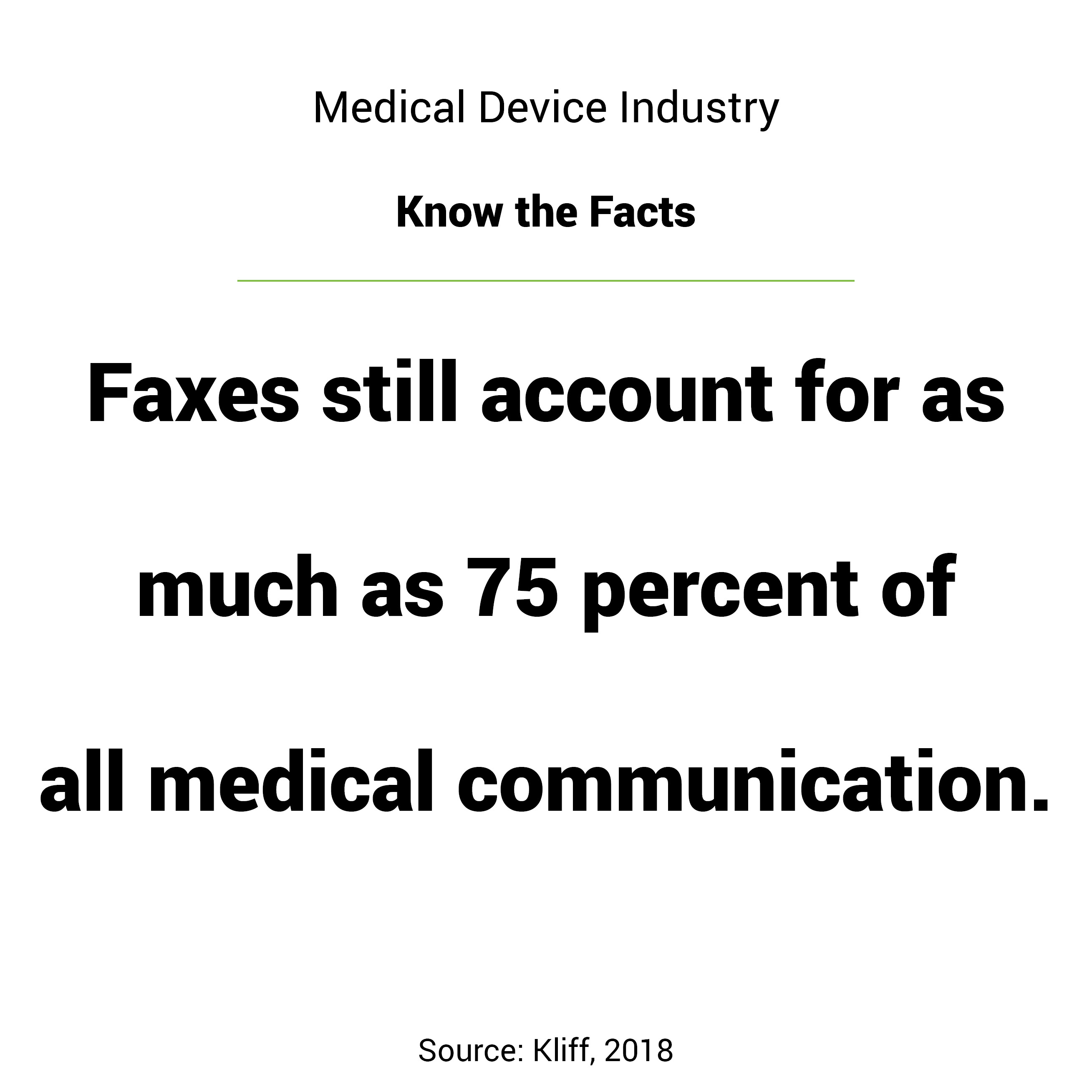
When it comes to electronic medical records, several words beginning with ‘F’ may come to mind.
You may be surprised to learn that, according to an article by Sarah Kliff, faxes still account for as much as 75 percent of all medical communication (Kliff, 2018). This is due to several factors. One of the main drivers is the lack of interoperability between competing electronic medical records systems.
So the fax machine survives, even though it is a dated technology widely despised by stakeholders throughout the healthcare spectrum.
Advantages to the fax machine include that it doesn’t require a login to access nor does it need months of training to operate. However, while these can be seen as benefits for end users, challenges arise when analyzing the security, traceability and confirmation of faxed documents.
Topics: Outsourcing Support, Medical Device Recall, Medical Device Recall Help, Med-Tech Industry Update, Medical Device Industry, Industry Trends, Industry Advancements, SMART recall program
5 Reasons to Join Novasyte’s Field Technical Team
Have you been considering a new career in 2019? The start of the year is a great time to explore different options as January marks the start of an active recruiting season for many companies. Novasyte is no exception.
As a leader in the med-tech space, Novasyte employs 1,000+ healthcare professionals across the U.S. and continues to recruit for new positions. One of the programs that has seen exponential growth is our Field Technical program.
Topics: Outsourcing Support, Med-Tech Industry Update, Medical Device Industry, Medical Device Field Action, S.M.A.R.T Programs, Industry Trends, SMART recall program
Novasyte is a Sponsor of the 5th Medical Device Postmarket Surveillance Conference
Novasyte is proud to announce we are a key sponsor at the upcoming 5th Medical Device Postmarket Surveillance Conference taking place this week in Alexandria, Virginia.
The conferences brings together executive leadership from a wide range of medical device categories, including capital equipment, diagnostic tests, active implantable devices as well as surgical tools.
Topics: Novasyte News, Medical Device Recall, Medical Device Recall Help, Med-Tech Industry Update, Medical Device Industry, Medical Device Field Action, S.M.A.R.T Programs, SMART recall program
For patients with diabetes, the challenge of managing their therapy can be alleviated with proper, quality education. For more than a decade, Novasyte has partnered with many of the largest, global medical device, diagnostic, and pharmaceutical organizations, providing specialized education with the highest-quality certified product trainers.
Topics: Novasyte News, Med-Tech Industry Update, Medical Device Industry, San Diego, CA, Industry Trends