Product recalls happen. Even to the most diligent organizations.
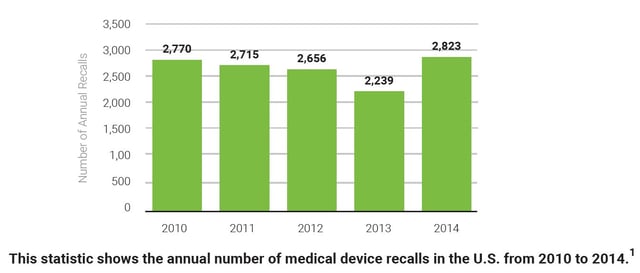
With the medical device landscape continually evolving, organizations must be agile and ready to respond to a recall at any moment. In looking at the annual number of medical device recalls in the graph below, one thing is known - recalls are not going away.
When a med-tech company gets notification of a potential field action, software upgrade or recall on one of their affected products, it often incites a level of internal alarm.
21 CFR 806.10 states manufacturers and importers are required to report a correction or removal of a product if it involves a risk to health. The report must be submitted to the FDA within 10 working days from the time the firm initiates the recall.² The associated urgency makes optimizing the recall management process critical to an organization’s success.
The high-sense-of-urgency combined with system limitations (many medical device OEMs rely on monolithic ERP systems), often leave companies with:
- A disrupted sales force
- Internal bandwidth issues preventing quick scalability
- Logistical challenges around tracking thousands of packages
- Warehouse limitations
- Brand degradation
- An approaching deadline
- Escalating, out-of-control costs with limited visibility until project end
This pressure mounts when the traditional internal recall process includes “talking to Sarah down the hall who completed a recall last year,” reviewing the customer data across multiple spreadsheets (often having varying degrees of accuracy), and relying on the traditional paper form notification and fax return protocol for consignee acknowledgment.
Upon launching a recall, the pressures often can shift to the following concerns, all with the compounding stress of completing the recall with accuracy and speed:
- Data capture and reporting
- The potential for trunk stock
- Managing adverse events
- Records retention (as dictated by an organization’s record control and retention policy)
- The potential need for, and strategy around, field-team deployment
Individuals who have previously experienced “recall chaos” may want to consider an outsourced partner to manage the recall, including real-time activity tracking on all actions from consignee notification to removal and warehousing.
Novasyte — the outsourced contract clinical, field technical and recall experts in the med-tech space —managed a large number of recalls following the traditional method (i.e. spreadsheets and fax machines), before developing the S.M.A.R.T. Program. To date, Novasyte has managed more than ten FDA-notified programs on the S.M.A.R.T. Platform, where their clients’ have received formal FDA recall termination approval in as quickly as three days.
With the recent increase in scrutiny with FDA Recall Coordinators managing specialized industries, including the Division of Medical Device and Radiological Health, it is more important now than ever to be tech-forward and strategic about the way your FDA recalls are managed.³ It is important to have the strategy and system not only work, but work for the organization.
To learn more how to increase the speed and accuracy of a recall, download our white paper to learn:
- Strategies for maintaining accurate consignee data
- Strategies for developing a methodical approach for a field strategy
- The risks associated with a de-centralized database, and why centralizing it is a necessity
- Case study outlining the S.M.A.R.T. method for managing medical device recalls
Sources:
1. Number of medical device recalls in the U.S. from 2010 to 2014. (2017). [Graph illustration]. Statistica. Retrieved from https://www.statista.com/statistics/618125/medical-device-recalls-in-the-us-by-number/
2. FDA Department of Health and Human Services Medical Devices; Reports of Corrections and Removals, 21 C.F.R. § 806.10 (2017). Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=806.10
3. U.S. Food & Drug Administration. (2017, October 24). ORA Recall Coordinators. Retrieved from https://www.fda.gov/safety/recalls/industryguidance/ucm129334.htm